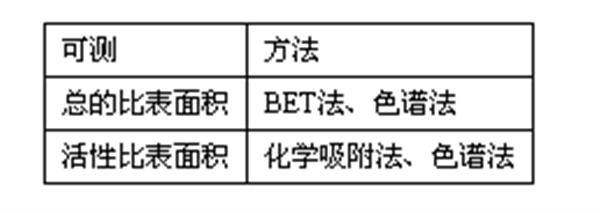
 3. BET adsorption isotherm equation - BET specific surface (currently recognized as the standard method for measuring solid specific surface)
3. BET adsorption isotherm equation - BET specific surface (currently recognized as the standard method for measuring solid specific surface)  (2) Common methods for measuring the amount of gas adsorbed: 1. The volumetric method measures the difference in volume between a known amount of gas before and after adsorption, thereby instantly calculating the amount of gas adsorbed. The catalyst sample is subjected to a degassing treatment before the adsorption operation, and then an adsorption operation is performed. 2. The principle of the gravimetric gravimetric method is to weigh the weight of the gas adsorbed by the catalyzed sample by a specially designed method. This method adopts a quartz spring scale with high sensitivity, and directly measures the gas adsorption amount from the elongation after the sample adsorbs a trace amount of gas. The quartz spring scale is pre-calibrated. In addition to measuring the amount of adsorption, other operations are consistent with the volumetric method. The gravimetric method can measure several samples at the same time (depending on the number of tubes in the sample tube), so it has high working efficiency. However, limited to the sensitivity and strength of quartz springs, the accuracy of measurement is much lower than that of volumetric method, so it is usually used for the determination of samples with specific surface area greater than 50m2. 3. Gas chromatography The BET volume method and gravimetric method above require high vacuum devices. Moreover, a long time degassing treatment is performed before measuring the amount of adsorption of the sample. Gas chromatography, which was developed not long ago, measures the specific surface area of ​​the catalyst, does not require a high vacuum device, and is fast in measurement and high in sensitivity, making it more suitable for factory use. When measuring the specific surface area by chromatography, the stationary phase is the measured solid itself (ie, the adsorbent is the catalyst to be tested), the carrier gas can be selected from Nz, Hz, etc., and the adsorbent can be selected from substances that are volatile and have no chemical reaction with the solid to be tested. Such as CeHe, CCld, CH: OH, etc. 4. Separate determination of different specific surface areas of complex catalysts The above method for determining the specific surface area based on the physical adsorption principle can only measure the total surface area of ​​the catalyst, and cannot determine the specific surface area of ​​different components (such as active metals). Therefore, selective chemisorption is often used to determine the surface area occupied by the different components. The chemical adsorption of a gas on the surface of a catalyst differs from physical adsorption in that it has properties similar to or close to that of a chemical reaction, and thus has the ability to select a certain surface of the catalyst. There is no general method suitable for determining the surface area of ​​various catalyst components, but experiments must be conducted to find gases that are only chemically adsorbed to one component under the same conditions and inert to the other components, or the same gas in these Chemisorption can occur on the components, but the degree of adsorption varies, and can also be used to determine the surface area of ​​the different components. However, due to the complexity of chemisorption, only a few types of catalysts are currently available for successful measurements. (1) Measurement of Pt Surface Area Supported on Al2O3 or SiO2-A120 In many supported metal platinum catalysts, the surface of the catalyst is usually not entirely covered by Pt. For Pt/A12O and Pt/SiO2-A120 catalysts, to know the surface area of ​​Pt exposed on the support, it can be determined by chemisorption of H2, O2 or CO gas on platinum. At the temperature of chemisorption, these gases do not actually chemically interact with the A120 or SiO 2 -A 12 O support. The catalyst sample is subjected to a temperature-raised degassing process prior to chemical adsorption. The purpose of the treatment is to obtain a clean platinum surface. The degassing treatment is carried out under conditions of heating and vacuuming. The higher the temperature and vacuum, the more complete the degassing. However, the temperature should not be too high to prevent the platinum grains from being sintered. 1 chemical adsorption of hydrogen. Experiments have shown that under the appropriate conditions, when the chemisorption of hydrogen on the catalyst Pt/Al2O reaches saturation, each platinum atom on the surface adsorbs a hydrogen atom, that is, the ratio of H/Pt is equal to 1. Therefore, by selecting an appropriate chemisorption condition and measuring the amount of saturated adsorption of hydrogen in a certain amount of a known specific surface area catalyst, the number of platinum atoms exposed on the surface can be calculated. The platinum atomic number is multiplied by its atomic cross-sectional area to obtain the surface area of ​​platinum. 1 Hydrogen oxygen titration. In the hydrogen-oxygen titration method, the Pt/A12O catalyst is first adsorbed to oxygen in a greenhouse, and then hydrogen is adsorbed. Hydrogen and adsorbed oxygen combine to form water, and the generated water is absorbed. The surface area of ​​platinum was calculated from the amount of hydrogen consumed and further based on O/Pt=1. Some people think that the accuracy of the results obtained by this method is higher than that of H2 or 02. (2) Measurement of surface area of ​​copper oxide and cuprous oxide Determination of different surfaces of a complex catalyst requires selection of a special method depending on the nature of the catalyst. In the copper catalyst used for the oxidation reaction, copper oxide and cuprous oxide are in a dynamic equilibrium which varies with external conditions. The basis for determining the CuO-Cu2O system is based on the different chemisorption capacities of the two components for oxygen and carbon monoxide. That is, CuO is chemically adsorbed with CO, Cu2O and 02. Before the measurement of the copper catalyst sample, the adsorption amount on the 1 m 2 surface of CuO and Cu 2 O was measured in advance as a comparison standard. At 20 ° C and 0.533 - 0.80 kPa, the amount of oxygen chemically adsorbed on CuO was 0.030 cm 3 /m 2 , and the amount of adsorbed CO was 0.060 cm 3 /m 2 . In the determination of the surface of CuO and Cu20 in the copper catalyst, it is necessary to carry out the chemical adsorption experiments of 02 and CO respectively, according to the total adsorption amount of 02 and CO on the same mass of the catalyst, such as S1 and S2, respectively, the surface areas of CuO and Cu20, Then the following binary joint equation V(0 2)=0.030S 1 ten 0.114S 2 V(CO)=0.014S 1 ten 0.060 S 2 can be established, where V(O 2) and V(CO) are respectively The volume of O 2 and CO adsorbed on the same mass of the catalyst, cm 2 /g. Solve the equation
(2) Common methods for measuring the amount of gas adsorbed: 1. The volumetric method measures the difference in volume between a known amount of gas before and after adsorption, thereby instantly calculating the amount of gas adsorbed. The catalyst sample is subjected to a degassing treatment before the adsorption operation, and then an adsorption operation is performed. 2. The principle of the gravimetric gravimetric method is to weigh the weight of the gas adsorbed by the catalyzed sample by a specially designed method. This method adopts a quartz spring scale with high sensitivity, and directly measures the gas adsorption amount from the elongation after the sample adsorbs a trace amount of gas. The quartz spring scale is pre-calibrated. In addition to measuring the amount of adsorption, other operations are consistent with the volumetric method. The gravimetric method can measure several samples at the same time (depending on the number of tubes in the sample tube), so it has high working efficiency. However, limited to the sensitivity and strength of quartz springs, the accuracy of measurement is much lower than that of volumetric method, so it is usually used for the determination of samples with specific surface area greater than 50m2. 3. Gas chromatography The BET volume method and gravimetric method above require high vacuum devices. Moreover, a long time degassing treatment is performed before measuring the amount of adsorption of the sample. Gas chromatography, which was developed not long ago, measures the specific surface area of ​​the catalyst, does not require a high vacuum device, and is fast in measurement and high in sensitivity, making it more suitable for factory use. When measuring the specific surface area by chromatography, the stationary phase is the measured solid itself (ie, the adsorbent is the catalyst to be tested), the carrier gas can be selected from Nz, Hz, etc., and the adsorbent can be selected from substances that are volatile and have no chemical reaction with the solid to be tested. Such as CeHe, CCld, CH: OH, etc. 4. Separate determination of different specific surface areas of complex catalysts The above method for determining the specific surface area based on the physical adsorption principle can only measure the total surface area of ​​the catalyst, and cannot determine the specific surface area of ​​different components (such as active metals). Therefore, selective chemisorption is often used to determine the surface area occupied by the different components. The chemical adsorption of a gas on the surface of a catalyst differs from physical adsorption in that it has properties similar to or close to that of a chemical reaction, and thus has the ability to select a certain surface of the catalyst. There is no general method suitable for determining the surface area of ​​various catalyst components, but experiments must be conducted to find gases that are only chemically adsorbed to one component under the same conditions and inert to the other components, or the same gas in these Chemisorption can occur on the components, but the degree of adsorption varies, and can also be used to determine the surface area of ​​the different components. However, due to the complexity of chemisorption, only a few types of catalysts are currently available for successful measurements. (1) Measurement of Pt Surface Area Supported on Al2O3 or SiO2-A120 In many supported metal platinum catalysts, the surface of the catalyst is usually not entirely covered by Pt. For Pt/A12O and Pt/SiO2-A120 catalysts, to know the surface area of ​​Pt exposed on the support, it can be determined by chemisorption of H2, O2 or CO gas on platinum. At the temperature of chemisorption, these gases do not actually chemically interact with the A120 or SiO 2 -A 12 O support. The catalyst sample is subjected to a temperature-raised degassing process prior to chemical adsorption. The purpose of the treatment is to obtain a clean platinum surface. The degassing treatment is carried out under conditions of heating and vacuuming. The higher the temperature and vacuum, the more complete the degassing. However, the temperature should not be too high to prevent the platinum grains from being sintered. 1 chemical adsorption of hydrogen. Experiments have shown that under the appropriate conditions, when the chemisorption of hydrogen on the catalyst Pt/Al2O reaches saturation, each platinum atom on the surface adsorbs a hydrogen atom, that is, the ratio of H/Pt is equal to 1. Therefore, by selecting an appropriate chemisorption condition and measuring the amount of saturated adsorption of hydrogen in a certain amount of a known specific surface area catalyst, the number of platinum atoms exposed on the surface can be calculated. The platinum atomic number is multiplied by its atomic cross-sectional area to obtain the surface area of ​​platinum. 1 Hydrogen oxygen titration. In the hydrogen-oxygen titration method, the Pt/A12O catalyst is first adsorbed to oxygen in a greenhouse, and then hydrogen is adsorbed. Hydrogen and adsorbed oxygen combine to form water, and the generated water is absorbed. The surface area of ​​platinum was calculated from the amount of hydrogen consumed and further based on O/Pt=1. Some people think that the accuracy of the results obtained by this method is higher than that of H2 or 02. (2) Measurement of surface area of ​​copper oxide and cuprous oxide Determination of different surfaces of a complex catalyst requires selection of a special method depending on the nature of the catalyst. In the copper catalyst used for the oxidation reaction, copper oxide and cuprous oxide are in a dynamic equilibrium which varies with external conditions. The basis for determining the CuO-Cu2O system is based on the different chemisorption capacities of the two components for oxygen and carbon monoxide. That is, CuO is chemically adsorbed with CO, Cu2O and 02. Before the measurement of the copper catalyst sample, the adsorption amount on the 1 m 2 surface of CuO and Cu 2 O was measured in advance as a comparison standard. At 20 ° C and 0.533 - 0.80 kPa, the amount of oxygen chemically adsorbed on CuO was 0.030 cm 3 /m 2 , and the amount of adsorbed CO was 0.060 cm 3 /m 2 . In the determination of the surface of CuO and Cu20 in the copper catalyst, it is necessary to carry out the chemical adsorption experiments of 02 and CO respectively, according to the total adsorption amount of 02 and CO on the same mass of the catalyst, such as S1 and S2, respectively, the surface areas of CuO and Cu20, Then the following binary joint equation V(0 2)=0.030S 1 ten 0.114S 2 V(CO)=0.014S 1 ten 0.060 S 2 can be established, where V(O 2) and V(CO) are respectively The volume of O 2 and CO adsorbed on the same mass of the catalyst, cm 2 /g. Solve the equation  5. Specific surface of the specific surface (<1m 2 / g) sample Determination of the lower limit of the surface area of ​​the low temperature nitrogen adsorption method, generally the volume of the gas adsorbate in the sample tube of 1 m 2 / g (standard state) minus the sample tube The volume of the unadsorbed gas (standard state). In the case of using nitrogen as the adsorbate, the measurement of the amount of adsorption will result in a large error in the sample with a small surface area. Because the adsorption amount is small at this time, and the nitrogen saturated vapor pressure as the adsorbate at liquid nitrogen temperature is close to the atmospheric pressure, the amount of nitrogen remaining in the sample tube after the adsorption equilibrium is reached under a certain relative pressure in the experimental range. Still large, the amount of total nitrogen that is initially transferred to the sample tube (before adsorption) is comparable and not easily calibrated. The biggest advantage of the ruthenium adsorption method is that the saturated vapor pressure of ruthenium at liquid nitrogen temperature is only about 2 mmHg. Therefore, in the measurement range of the adsorption isotherm, the unadsorbed ruthenium remaining in the dead space after the adsorption equilibrium is reached. The change in gas volume is large and can be measured accurately, so helium is suitable for the determination of low specific surface solids. 6. Determination of active surface area The total surface area of ​​the adsorbent determined by the BET method, and usually a part of it, is active. This part is called active surface. The area of ​​active surface can be determined by "selective chemisorption" method, such as surface hydrogen and oxygen. Titration method. Many high specific surface area adsorbents are pore-shaped, and for such materials, the external specific surface and the inner surface are often distinguished. The outer surface refers to the peripheral area of ​​the individual particles or agglomerates. But because at the atomic scale, the surface of a solid is rarely smooth, it is difficult to define it accurately. It is generally agreed that the outer surface includes all protrusions and those surfaces having a width greater than the depth of the crack. The inner surface is the wall of all cracks, holes, holes of depth greater than the width. 7. Langmuir adsorption isotherm equation - Langmuir specific surface (1) Langmuir theoretical model The surface of the adsorbent is uniform, the energy of each adsorption center is the same; the interaction between adsorbed particles can be neglected; the adsorption particles collide with the empty adsorption center is possible Adsorbed, an adsorbed particle occupies only one adsorption center, and the adsorption is single-layered and positioned; under certain conditions, the adsorption rate is equal to the desorption rate, and the adsorption equilibrium is reached. (2) Isotherm equation adsorption rate: raâˆ(1-θ)P ra=ka(1-θ)P desorption rate rdâˆÎ¸ rd=kdθ reaches adsorption equilibrium: ka(1-θ)P=kdθ where θ = Va / Vm (Va - adsorption amount of gas adsorbate; Vm - monolayer saturated adsorption capacity, mol / g), which is the fraction of the surface of the adsorbent covered by gas molecules, that is, the coverage. Let B = ka / kd, then: θ = Va / Vm = BP / (1 + BP), finishing can be obtained: P / V = ​​P / Vm + 1 / BVm plotted as P / V ~ P, is a straight line, According to the slope and intercept, the B and Vm values ​​can be obtained (the reciprocal of the slope is Vm), so the adsorbent has a specific surface area of: Sg = Vm · A · σ mA - Avogadro constant (6.023 x 1023 / mol) σm - an adsorption The cross-sectional area of ​​the molecular molecule (N2 is 16.2x10-20 m2), that is, the area occupied by each nitrogen molecule on the surface of the adsorbent. This formula applies to: substances containing pure micropores; chemical adsorption.
5. Specific surface of the specific surface (<1m 2 / g) sample Determination of the lower limit of the surface area of ​​the low temperature nitrogen adsorption method, generally the volume of the gas adsorbate in the sample tube of 1 m 2 / g (standard state) minus the sample tube The volume of the unadsorbed gas (standard state). In the case of using nitrogen as the adsorbate, the measurement of the amount of adsorption will result in a large error in the sample with a small surface area. Because the adsorption amount is small at this time, and the nitrogen saturated vapor pressure as the adsorbate at liquid nitrogen temperature is close to the atmospheric pressure, the amount of nitrogen remaining in the sample tube after the adsorption equilibrium is reached under a certain relative pressure in the experimental range. Still large, the amount of total nitrogen that is initially transferred to the sample tube (before adsorption) is comparable and not easily calibrated. The biggest advantage of the ruthenium adsorption method is that the saturated vapor pressure of ruthenium at liquid nitrogen temperature is only about 2 mmHg. Therefore, in the measurement range of the adsorption isotherm, the unadsorbed ruthenium remaining in the dead space after the adsorption equilibrium is reached. The change in gas volume is large and can be measured accurately, so helium is suitable for the determination of low specific surface solids. 6. Determination of active surface area The total surface area of ​​the adsorbent determined by the BET method, and usually a part of it, is active. This part is called active surface. The area of ​​active surface can be determined by "selective chemisorption" method, such as surface hydrogen and oxygen. Titration method. Many high specific surface area adsorbents are pore-shaped, and for such materials, the external specific surface and the inner surface are often distinguished. The outer surface refers to the peripheral area of ​​the individual particles or agglomerates. But because at the atomic scale, the surface of a solid is rarely smooth, it is difficult to define it accurately. It is generally agreed that the outer surface includes all protrusions and those surfaces having a width greater than the depth of the crack. The inner surface is the wall of all cracks, holes, holes of depth greater than the width. 7. Langmuir adsorption isotherm equation - Langmuir specific surface (1) Langmuir theoretical model The surface of the adsorbent is uniform, the energy of each adsorption center is the same; the interaction between adsorbed particles can be neglected; the adsorption particles collide with the empty adsorption center is possible Adsorbed, an adsorbed particle occupies only one adsorption center, and the adsorption is single-layered and positioned; under certain conditions, the adsorption rate is equal to the desorption rate, and the adsorption equilibrium is reached. (2) Isotherm equation adsorption rate: raâˆ(1-θ)P ra=ka(1-θ)P desorption rate rdâˆÎ¸ rd=kdθ reaches adsorption equilibrium: ka(1-θ)P=kdθ where θ = Va / Vm (Va - adsorption amount of gas adsorbate; Vm - monolayer saturated adsorption capacity, mol / g), which is the fraction of the surface of the adsorbent covered by gas molecules, that is, the coverage. Let B = ka / kd, then: θ = Va / Vm = BP / (1 + BP), finishing can be obtained: P / V = ​​P / Vm + 1 / BVm plotted as P / V ~ P, is a straight line, According to the slope and intercept, the B and Vm values ​​can be obtained (the reciprocal of the slope is Vm), so the adsorbent has a specific surface area of: Sg = Vm · A · σ mA - Avogadro constant (6.023 x 1023 / mol) σm - an adsorption The cross-sectional area of ​​the molecular molecule (N2 is 16.2x10-20 m2), that is, the area occupied by each nitrogen molecule on the surface of the adsorbent. This formula applies to: substances containing pure micropores; chemical adsorption. IDC Type
IDC Type
ATKCONN ELECTRONICS CO., LTD , https://www.atkconn.com